Copyright © . Transposon Therapeutics, Inc. All rights reserved. Privacy Policy Terms of Use
Transformational therapies for neurodegenerative and aging-related diseases

✕
Science
Science
Our mission is to develop innovative treatments for LINE-1 associated neurodegenerative disease.
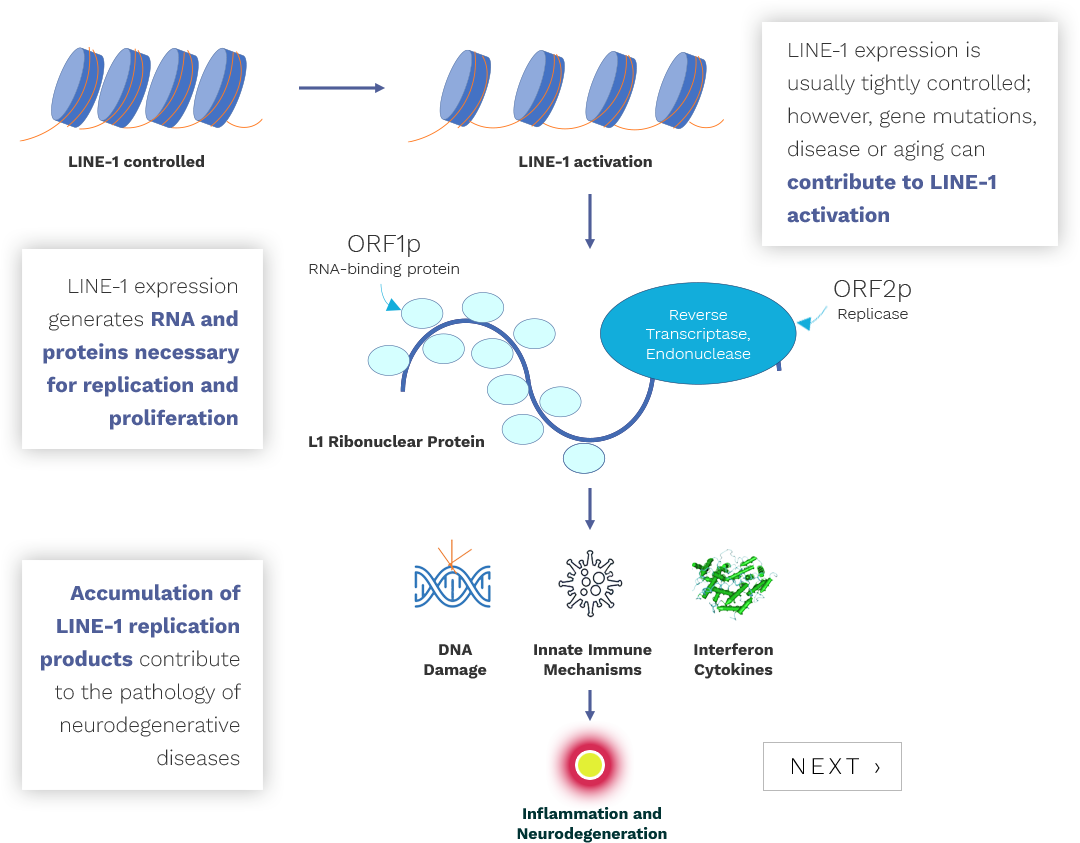
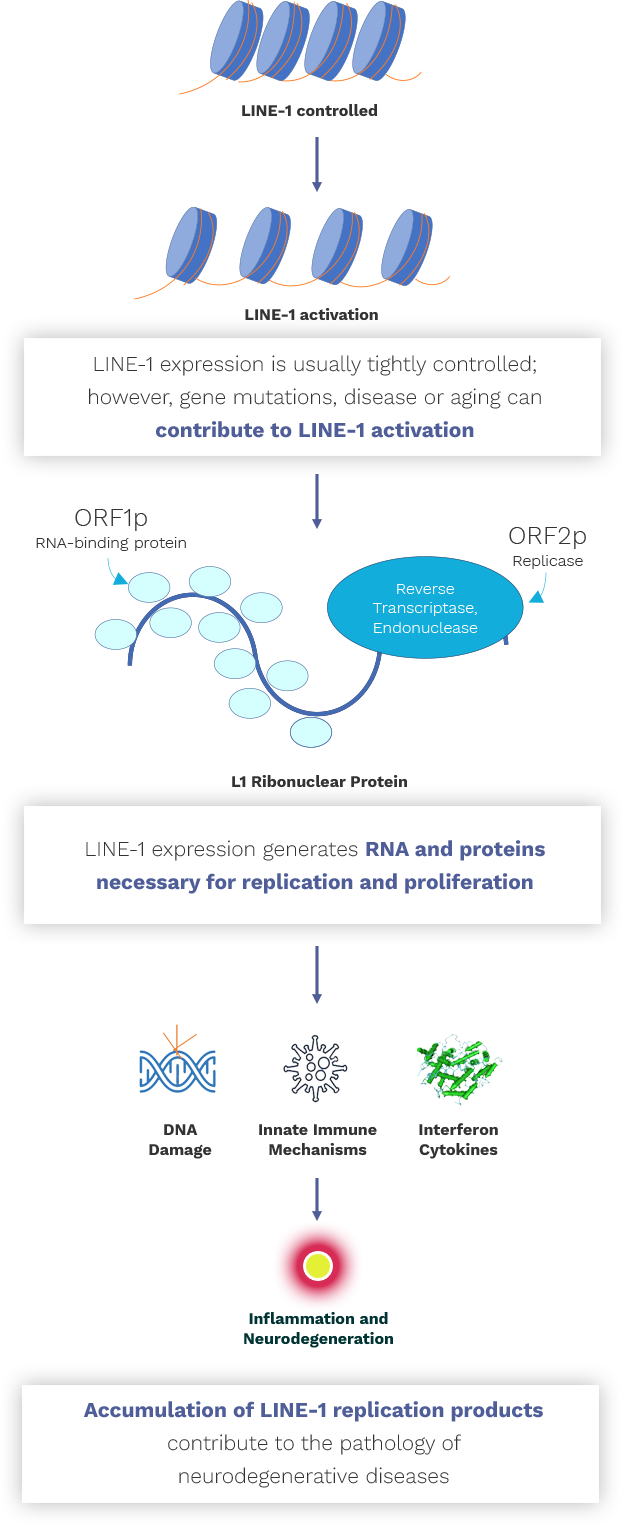
Almost half of our genome is occupied by virus-like elements called “retrotransposons” which have been introduced into our genome over millions of years. Retrotransposons are mobile DNA elements that use a “reverse transcriptase” enzyme to self-replicate and proliferate. Since these activities can be damaging to our cells, our bodies maintain strict control over retrotransposon expression.
Today, the only retrotransposons in our genome that are capable of becoming active are human-specific LINE-1 elements. Abnormal proteins that lead to neurodegenerative diseases, such as tau and TDP-43, can increase the expression of these LINE-1 elements, initiating a cascade of events in the neuron that leads to its dysfunction and death.
Transposon Therapeutics, Inc. is developing inhibitors of LINE-1 reverse transcriptase and other mediators of LINE-1 pathology for the treatment of neurodegenerative disease.
More
Transposon Therapeutics, Inc. is developing inhibitors of LINE-1 reverse transcriptase and other mediators of LINE-1 pathology for the treatment of neurodegenerative disease.
More
✕
Approach
Transposon is developing nucleoside inhibitors of LINE-1 reverse transcriptase (NRTI) and other mediators of LINE-1 pathology (e.g., Protein Kinase R inhibitors [PKR]) for the treatment of neurodegenerative disease.
Transposon Pipeline
| Asset | Indications | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
| Orphan Diseases | ||||||
| TPN-101 | Progressive Supranuclear Palsy (PSP) | In planning | ||||
| TPN-101 | Amyotrophic Lateral Sclerosis (ALS) / Frontotemporal Dementia (FTD) | In planning | ||||
| TPN-101 | Aicardi-Goutières Syndrome (AGS) | Ongoing | ||||
| New Indications | ||||||
| TPN-101 | Alzheimer's Disease & Others | In planning | ||||
| Novel NRTIs | To be determined | |||||
| Novel PKRi | Disorders of cognition | |||||
✕
Approach
TPN-101
The most potent known inhibitor of LINE-1 reverse transcriptase is currently under study in two Phase 2a clinical trials.
Our lead reverse transcriptase inhibitor, TPN-101, is the most potent known inhibitor of LINE-1 reverse transcriptase and has excellent systemic and brain bioavailability with once daily oral dosing. TPN-101, also known as censavudine, was well tolerated in a 48-week study of over 220 HIV patients.
The current TPN-101 Phase 2a clinical development program is designed to establish proof-of-concept for treating several neurodegenerative diseases with high unmet medical need and evidence for involvement of LINE-1 reverse transcriptase. Biomarkers will be used to measure changes in inflammation and neurodegeneration to establish proof-of-concept along with safety and clinical efficacy assessments.
Expanded Access Policy PSP ALS/FTD AGS
The current TPN-101 Phase 2a clinical development program is designed to establish proof-of-concept for treating several neurodegenerative diseases with high unmet medical need and evidence for involvement of LINE-1 reverse transcriptase. Biomarkers will be used to measure changes in inflammation and neurodegeneration to establish proof-of-concept along with safety and clinical efficacy assessments.
Expanded Access Policy PSP ALS/FTD AGS
✕
PSP
Progressive Supranuclear Palsy (PSP)
Why TPN-101
PSP is a primary 4R tauopathy, that is, a disease associated with abnormal accumulation of neurotoxic tau proteins. In the PSP brain, LINE-1 expression is increased and correlates with tau neurofibrillary tangles and interferon-related gene expression. Preclinical evidence shows blocking LINE-1 reverse transcription with TPN-101 mitigates neurodegeneration.
PSP is a primary 4R tauopathy, that is, a disease associated with abnormal accumulation of neurotoxic tau proteins. In the PSP brain, LINE-1 expression is increased and correlates with tau neurofibrillary tangles and interferon-related gene expression. Preclinical evidence shows blocking LINE-1 reverse transcription with TPN-101 mitigates neurodegeneration.
Clinical Trial Information
In the US, we completed a Phase 2a study of TPN-101 in patients with PSP; the study included a 24-week double-blind, placebo-controlled period followed by a 24-week open-label extension period.
For More Information
ClinicalTrials.gov
In the US, we completed a Phase 2a study of TPN-101 in patients with PSP; the study included a 24-week double-blind, placebo-controlled period followed by a 24-week open-label extension period.
For More Information
ClinicalTrials.gov

Approach › TPN-101
PSP
Progressive Supranuclear Palsy
A devastating orphan disease, with mean survival of 6 to 7 years, characterized by early postural instability and falls, vertical gaze palsy, akinesia, rigidity, pseudobulbar palsy, and frontal dysfunction with cognitive and behavioral changes.
More
More
✕
ALS/FTD
Amyotrophic Lateral Sclerosis (ALS) / Frontotemporal Dementia (FTD)
Why TPN-101
TDP-43 proteinopathies are found in most ALS patients (over 90%) and approximately 40% of FTD patients. Loss of nuclear TDP-43 leading to increased LINE-1 expression and reverse transcription is enriched in FTD or ALS patients with mutation of the C9ORF72 gene and associated with neuroinflammation and induction of Type 1 interferons. Preclinical evidence shows blocking LINE-1 reverse transcription with TPN-101 mitigates neurotoxic pathology.
TDP-43 proteinopathies are found in most ALS patients (over 90%) and approximately 40% of FTD patients. Loss of nuclear TDP-43 leading to increased LINE-1 expression and reverse transcription is enriched in FTD or ALS patients with mutation of the C9ORF72 gene and associated with neuroinflammation and induction of Type 1 interferons. Preclinical evidence shows blocking LINE-1 reverse transcription with TPN-101 mitigates neurotoxic pathology.
Clinical Trial Information
To evaluate the therapeutic value of TPN-101 for ALS or FTD, our initial clinical study will focus on patients with C9ORF72 mutations. Enrollment is complete in our Phase 2a study of TPN-101. It is a 24-week double-blind, placebo-controlled study followed by a 24-week open-label extension period.
For More Information
To evaluate the therapeutic value of TPN-101 for ALS or FTD, our initial clinical study will focus on patients with C9ORF72 mutations. Enrollment is complete in our Phase 2a study of TPN-101. It is a 24-week double-blind, placebo-controlled study followed by a 24-week open-label extension period.
For More Information
|
AFTD Website FTD Registry NEALS Website |
NEALS Video: Dr. Merit Cudkowicz National ALS Registry ClinicalTrials.gov |

Approach › TPN-101
ALS
Amyotrophic Lateral SclerosisFTD
Frontotemporal DementiaThe median survival for ALS is 3 years and 9 years for FTD. When ALS and FTD present as co-morbidities, survival is markedly reduced.
ALS is an orphan disease characterized by progressive muscle weakness and loss of ability to speak, eat, move or breathe.
FTD is a progressive frontal / temporal cortex disease associated with behavior and personality changes, emotional problems, difficulty communicating, working or walking.
More
FTD is a progressive frontal / temporal cortex disease associated with behavior and personality changes, emotional problems, difficulty communicating, working or walking.
More
✕
AGS
Aicardi Goutières Syndrome (AGS)
Why TPN-101
Genetic mutations of LINE-1 repressing enzymes (e.g., TREX-1, RNASEH2) cause AGS and result in uncontrolled LINE-1 activation with severe interferon-1 autoimmune reaction. Clinical validation of LINE-1 reverse transcriptase as a therapeutic target was demonstrated in a study of AGS patients receiving reverse transcriptase inhibitors (abacavir, lamivudine and zidovudine) which lowered interferon scores and increased blood flow to the brain.
Genetic mutations of LINE-1 repressing enzymes (e.g., TREX-1, RNASEH2) cause AGS and result in uncontrolled LINE-1 activation with severe interferon-1 autoimmune reaction. Clinical validation of LINE-1 reverse transcriptase as a therapeutic target was demonstrated in a study of AGS patients receiving reverse transcriptase inhibitors (abacavir, lamivudine and zidovudine) which lowered interferon scores and increased blood flow to the brain.
Clinical Trial Information
An open-label, Phase 2a study of TPN-101 in AGS patients is open for enrollment. Cohort 1, comprised of adult patients, has been completed. The study is recruiting patients aged 1 to 17 years to fill the remaining cohorts. The study is being conducted in France and Italy.
For More Information
ClinicalTrials.gov
An open-label, Phase 2a study of TPN-101 in AGS patients is open for enrollment. Cohort 1, comprised of adult patients, has been completed. The study is recruiting patients aged 1 to 17 years to fill the remaining cohorts. The study is being conducted in France and Italy.
For More Information
ClinicalTrials.gov

Approach › TPN-101
AGS
Aicardi Goutières Syndrome
AGS is an ultrarare severe pediatric disease generally presenting in infancy; many patients do not survive childhood. Neuronal degeneration causes massive brain damage and loss of developmental milestones beginning shortly after birth and can result in heart and muscle damage.
More
More
✕
Approach
Novel NRTIs
Next generation nucleoside reverse transcriptase inhibitors (NRTIs)Transposon-proprietary NRTIs will allow rapid exploration of new indications in which there is compelling evidence for LINE-1 related pathology.
Transposon employs informatics and pharmacology research to identify neurologic as well as age-related and autoimmune diseases associated with LINE-1 pathology. Transposon’s inhibitor discovery program has developed a robust pipeline of innovative next generation LINE-1 inhibitors which will be deployed to explore selected disease indications with high unmet medical needs.
✕
Approach
PKR
Protein Kinase RDownstream druggable targets in the LINE-1 pathway, such as Protein Kinase R (PKR), may be additive or synergistic with LINE-1 reverse transcriptase inhibitor treatment.
Protein kinase R (PKR) is elevated in the cerebrospinal fluid of Alzheimer’s Disease patients, and the degree of elevation correlates with severity of cognitive impairment. Knock-out of the PKR gene in mice leads to improved cognition and memory formation. Small molecule PKR inhibitors have been shown to improve memory and cognition in mouse models.
Our PKR inhibitor discovery program evaluates innovative small molecules for the treatment of compromised cognition and memory associated with central nervous system diseases.
Our PKR inhibitor discovery program evaluates innovative small molecules for the treatment of compromised cognition and memory associated with central nervous system diseases.

Dennis Podlesak
CEO and Chair
CEO and Chair
Dennis is CEO / Managing Partner of Axcelius LLC and advisory partner to Domain Associates. His board service or CEO leadership has resulted in many successful exits (Tobira/Allergan, Avanir/Otsuka, Corthera/ Novartis, Rightcare Solutions/Cardinal Health, Calixa/ Cubist, Cerexa/Forest, Peninsula/J&J). Previously, he held executive positions at Novartis, Allergan, and SKB.

Eckard Weber, MD
Chief Innovation Officer
Chief Innovation Officer
Bringing > 30 years experience, Eckard specializes in creating biopharm companies, many of which have been acquired or listed on public markets. He is venture partner at Canaan Partners and an advisory partner at Domain Associates. He is an inventor/co-inventor of numerous patents and has published > 130 papers.

Michael Cordingley, PhD
Chief Scientific Officer
Chief Scientific Officer
With > 30 years of experience across multiple therapeutic areas, Michael has held R&D positions at Merck and BMS prior to joining BI as Preclinical TA Head Virology and Infectious Disease and SVP, R&D Site Head, and member of R&D international leadership team. He has held positions at the NCI, NIH, and McGill University.

Andrew Satlin, MD
Chief Medical Officer
Chief Medical Officer
Andy brings > 20 years of drug development experience and a focus on novel clinical and statistical methods for neurodegenerative disorders. He was EVP and CMO at Intra-Cellular Therapies, held positions at Eisai and Novartis, and was Assistant Professor of Psychiatry at Harvard Medical School and Director of Geriatric Psychiatry at McLean Hospital.

Lynne Rollins
Chief Financial Officer
Chief Financial Officer
With > 40 years of experience in healthcare finance and business development, Lynne serves as CFO for several start-up pharmaceutical and medical device companies. Previously, she served 19 years with Baxter Healthcare Corporation with her latest position as VP Finance for the Cardiopulmonary Business.

Rick Orr
Head, BD and Corp Counsel
Head, BD and Corp Counsel
Rick brings > 20 years of operational, development and legal experience creating and building life science companies. He serves on the board of SiteOne Therapeutics, was CEO of Adynxx, and held executive leadership positions at Corthera, Calixa, Cerexa and Peninsula Pharmaceuticals. Previously, Rick was an attorney at Cooley LLP.

Walter Bee, PhD
SVP Preclinical & Biomarker Development
SVP Preclinical & Biomarker Development
Walter has > 30 years experience in nonclinical development and preclinical safety testing. He served as VP Toxicology and Regulatory Consulting and Global Head of Safety Assessment and Regulatory Affairs for QPS, LLC. Previously, he held positions with Halozyme, J&J, Scios, CoCensys, and several leading contract research organizations.

Mark Mugerditchian
VP Manufacturing
VP Manufacturing
Mark has > 42 years CMC experience. His broad technical background covers several formats of drug substance (small molecule synthesis and biotechnology) and drug product (SVP, LVP, Solid Oral Dose, inhalation, transdermal, diagnostic). Recent posts include PvP Biologics, Laguna Pharmaceuticals, Sequel, NovaMedica and NovaCardia.

Jay Soto
Head, Clinical Operations
Head, Clinical Operations
Jay brings > 20 years experience in development operations, AI and clinical strategy. He has a diverse background, focusing mainly on neurodegenerative disorders, oncology, immunology, and amyloid-related diseases. Prior roles include VP, Clinical Operations at Prothena as well as positions at Pharmacyclics, Janssen Alzheimer Immunotherapy, Elan, and Amgen.

Priya Jambhekar
Head, Regulatory
Head, Regulatory
Priya brings > 30 years experience in regulatory and quality affairs. She is experienced in early stage to late state development and post approval activities supporting drugs, biologics, medical devices and combination products in several therapeutic areas. Prior roles include head of regulatory affairs for Baxter Critical Care, Ethicon, Alkermis, Paramount Biosciences.

Ethan Geier, PhD
Senior Director, Research and Development
Senior Director, Research and Development
Ethan brings bioinformatic expertise and insight into neurodegenerative disease pathobiology to support the Company’s biomarker development and biomarker analysis for clinical programs. Previously, he was head of bioinformatics at PrimeFour Therapeutics, and a staff scientist at the University of California, San Francisco Memory and Aging Center. He also was President and Co-founder of Apricity Therapeutics where he led and coordinated preclinical discovery and development.
✕
Management Team
 Dennis Podlesak CEO and Chair |
 Eckard Weber, MD Chief Innovation Officer |
 Michael Cordingley, PhD Chief Scientific Officer |
 Andrew Satlin, MD Chief Medical Officer |
 Lynne Rollins Chief Financial Officer |
 Rick Orr Head, BD and Corp Counsel |
 Walter Bee, PhD SVP Preclinical & Biomarker Development |
 Mark Mugerditchian VP Manufacturing |
 Priya Jambhekar Head, Regulatory |
 Jay Soto Head, Clinical Operations |
 Ethan Geier, PhD Senior Director, Research and Development |
|
✕
Advisory Boards
Scientific Advisory Board
John Sedivy, PhD Chair
Brown University
Kathy Burns, MD PhD
DFCI, Harvard Med School
George Church, PhD
Harvard University
Josh Dubnau, PhD
SUNY at Stony Brook
Rusty Gage, PhD
Salk Institute
Molly Hammel, PhD
Cold Spring Harbor Lab
Clinical Advisory Board
Murali Doraiswamy, MD Chair
Duke University
Adam Boxer, MD
UCSF
Yanick Crow, MD, PhD
Univ. Edinburgh (AGS)
Merit Cudkowicz, MD
MGH
John Ravits, MD
UCSD

Dennis Podlesak
CEO and Chair
CEO and Chair
Dennis is CEO / Managing Partner of Axcelius LLC and advisory partner to Domain Associates. His board service or CEO leadership has resulted in many successful exits (Tobira/Allergan, Avanir/Otsuka, Corthera/ Novartis, Rightcare Solutions/Cardinal Health, Calixa/ Cubist, Cerexa/Forest, Peninsula/J&J). Previously, he held executive positions at Novartis, Allergan, and SKB.

Brent Ahrens
Director
Director
Brent is a general partner at Canaan focusing on biopharma & medtech investments, many resulting in successful acquisitions. Current Boards include Abyrx, EndoGastric Solutions, Grey Wolf, Pathios, Relievant MedSystems, and ThirtyFiveBio. Previously, Brent held roles at General Surgical Innovations, Ethicon, and IAP Research and has several surgical instrument patents.

Bill Harrington, MD
Director
Director
Bill is Managing Partner at Osage University Partners, a venture capital firm investing in university licensees and spinouts where he is responsible for life science investing. Previously, Bill was General Partner at Three Arch Partners. He has invested in > 80 life science companies and serves the boards of Hyalex Orthopedics, Neuros Medical, MediSix Therapeutics.

Daniel Hetu, MD
Director
Director
With 20+ years of investment banking, corporate development, and licensing experience, Daniel manages Lumira Ventures’ Montreal. His activities focus on building therapeutic and medical device companies that impact patient care. Prior to Lumira, Daniel was a senior executive at Shire and Biochem Pharma and an investment banker at a major Canadian Investment Bank.

Keith Katkin
Director
Director
Keith brings almost 30 years of pharmaceutical/biotech experience at large cap, small cap and start-up companies. Keith has significant experience in clinical development, sales and marketing as well as business development. After the successful sales of Avanir and Urovant Sciences, Keith now serves on the Board of Directors of Syndax, Emergent BioSolutions and Eledon.

John Pacifico
Director
Director
With 20+ years as venture capitalist and healthcare CEO, John brings a wide-range of experience to the Board. He is a General Partner at Canaan, founder & CEO of Abyrx and founder & chairman of the board at Kairuku. He serves the boards of Axcelius, PrimeFour, and Aceiss. Previously, he was President & CEO of ORTHOCON®. He is an inventor of 20+ issued or pending patents.

BT Slingsby, MD
Director
Director
Founder & Managing Partner at Catalys Pacific, BT is a physician-scientist and biotechnology entrepreneur leading company creation efforts for the firm. Previously, he founded the GHIT Fund – the world’s first public-private fund focused on development of new medicines for low- and middle-income countries. He is an inventor of multiple patents and author of over 50 scientific papers.

Eckard Weber, MD
Director and Chief Innovation Officer
Director and Chief Innovation Officer
Bringing > 30 years experience, Eckard specializes in creating biopharm companies, many of which have been acquired or listed on public markets. He is venture partner at Canaan Partners and an advisory partner at Domain Associates. He is an inventor/co-inventor of numerous patents and has published > 130 papers.
✕
Board of Directors
 Dennis Podlesak CEO and Chair |
 Brent Ahrens Director |
 Bill Harrington, MD Director |
 Daniel Hetu, MD Director |
 Keith Katkin Director |
 John Pacifico Director |
 BT Slingsby, MD Director |
 Eckard Weber, MD Director and Chief Innovation Officer |
|
Team
Transposon is powered by world renowned, pioneering scientists, experienced drug development experts with a collective record of over 30 NDA approvals, and repeat entrepreneurs.
✕